Solution
Safety means to eliminate all possibilities of dangers. It is particularly true for the mega Nankai Trough Earthquake. Currently we have the following dangers:
1. 19,000 tons of the spent nuclear fuel
2. 12 active nuclear reactors
3. High-level radioactive waste in Tokai
4. Plutonium in Monju
We are going to think about the solution to those problems assuming that the prediction by Ryo Tatsuki (The Economic Times [9]) is true.
1. 19,000 tons of the spent nuclear fuel
There are two ways to store the spent nuclear fuel, cooling pool and dry cask.
The minimum depth for the cooling pool is 6 meters, but usually it is 12 meters. The water has the function to cool the spent fuel and shield the radioactivity emitted from the fuel. The cooling pool also needs to detect the leaking of water. (Wikipedia (Spent fuel pool) [7]) Dry casks are sealed steel cylinders and store the spent fuel rods surrounded by inert gas.(Wikipedia (Dry cask storage) [1]) They can keep the spent fuel cool without electricity. The lifetime of the dry casks is several decades up to 100 years. The spent nuclear fuel retrieved from the nuclear reactor is most dangerous. However, in the cooling pool the radioactivity of the spent fuel decreases rapidly and after a few years it can be stored in the dry casks. The minimum cooling period in the pool before the dry cask storage is one year for the normal uranium fuel and three years for the MOX fuel (the uranium-plutonium mixed fuel). (United States Nuclear Regulatory Commission [2], Satoshi Ishikawa et al.[3]) The price of the dry cask is about 100 million – 200 million yen ( ~ 1 million – 2 million dollars) and each cask can store 10 – 15 tons of spent fuel.
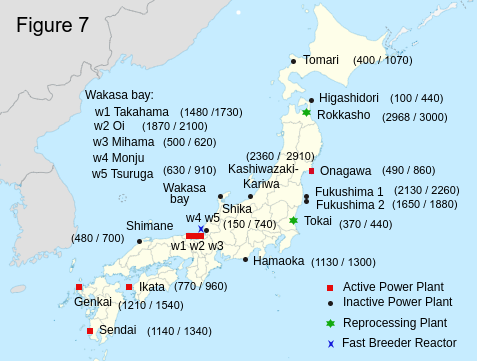 Figure 7 shows (the stored spent nuclear fuel / the storage capacity) (in ton unit) in the cooling pool in each nuclear power plant. In the cooling pool, the loss of electricity will cause the meltdown of the fuel within three days. Therefore the best solution for the storage of the spent nuclear fuel is to use the dry cask. However, a sufficient number of dry casks may not be available immediately. To survive the Nankai Trough Earthquake we may have to use cooling pools with secure electricity and without any risks of tsunami, such as in the north part of Japan and in foreign countries. The spent fuel in Ikata and Hamaoka needs to be stored in the dry casks or to be moved to safer places right now. The spent fuel in the other plants in the south part of Japan also need to be protected in the same way.
Figure 7 shows (the stored spent nuclear fuel / the storage capacity) (in ton unit) in the cooling pool in each nuclear power plant. In the cooling pool, the loss of electricity will cause the meltdown of the fuel within three days. Therefore the best solution for the storage of the spent nuclear fuel is to use the dry cask. However, a sufficient number of dry casks may not be available immediately. To survive the Nankai Trough Earthquake we may have to use cooling pools with secure electricity and without any risks of tsunami, such as in the north part of Japan and in foreign countries. The spent fuel in Ikata and Hamaoka needs to be stored in the dry casks or to be moved to safer places right now. The spent fuel in the other plants in the south part of Japan also need to be protected in the same way.
In Hamaoka nuclear power plant 1130 tons of spent nuclear fuel is still stored in the cooling pool. According to the homepage of Chubu Electric Power, they increased the earthquake resistance of the facilities up to 1200 gal and added the breakwater.(Chubu Electoric Power [4]) But those are completely useless. Hamaoka nuclear plant is located on the top of the three tectonic plates boundaries. The anticipated earthquake is much, much more fierce than 1200 gal. According to the new damage estimation (NHK [8]), the height of the tsunami in Hamaoka is 20 meters. Hamaoka nuclear plant is about 6 meters above sea level. Even if they consructed 14 meters breakwater, there are no guarantees that it will work. The liquefaction of the ground may occur beneath the breakwater and the actual tsunami may be 30-40 meters. What we have learned from the 2011 Tohoku earthquake is that the only way to survive from a tsunami is just running away from it. In Taro in Iwate, people had constructed a gigantic breakwater of 10 meters high and 2 kilometers long to defend their town, but the actual tsunami came far beyond the breakwater and destroyed it like pudding. Therefore the best solution is to put the spent nuclear fuel in the dry casks and move them to safe places beyond the reach of the tsunami.
2. 12 active nuclear reactors
Obviously we need to stop the reactor in Ikata now. According to the new damage estimation (NHK [8]), other reactors look relatively safe. However, we are facing the mega Nankai Trough Earthquake. Big earthquakes may occur at the Median Tectonic Line which is the north end of the strong earthquake fault zone (Figure 8, Cabinet office [9]). The Nankai Trough Earthquake may induce further sift along the fault lines in Wakasa bay and increase the volcanic activities near the Sendai nuclear power plant. The earthquake‐resistant limits of the nuclear power plants in Japan are only M6.5 (620 Gal – 1200 Gal). We should stop all 12 reactors before the Nankai Trough Earthquake occurs.
The nuclear power plants are the facilities to produce the billion-times intense radioactive substances after the one year operation and we have to take care of them for 100,000 years. We don’t know the true purpose of this insane conduct, but it is nothing but an unacceptable danger for us. The Japanese government advocates the nuclear fuel cycle. (Agency for Natural Resources and Energy [5]) However, Japan is located on the top of the boundary of four tectonic plates and it is impossible to safely carry out the geological disposal of radioactive waste. After 100,000 years, Japan on the map must be in a quite different shape. Therefore the cycle is not closed. In the situation of facing the Nankai Trough earthquoke, running the 12 nuclear reactors for the “nuclear fuel cycle” may result in a suicide attack against the whole human beings.
Assuming we were successful in stopping the 12 reactors, we continue our argument. The spent nuclear fuel retrieved from the nuclear reactor is too hot for the dry cask storage. We have to wait for at least one year for the normal uranium fuel and at least three years for the MOX fuel. The MOX fuel is currently used in four reactors; Genkai(unit 3), Ikata(unit 3), Takahama(unit 3, unit 4). Our solution is to transport the spent fuel retrieved from the reactors to the cooling pools in the safer places. Usually the spent fuel retrieved from a reactor is sent to the cooling pool next to the reactor. However it is possible to move the fuel to the cooling pool 100 km away from the reactor using the transportation casks. In Ikata and Hamaoka, 20 meters of tsunami is going to come over the nuclear plants. After removing the spent fuel, the reactors also need to be protected against the tsunami. We can move the reactors using the method called structure relocation. (YouTube [6])
3. High-level radioactive waste in Tokai
JAEA (Japan Atomic Energy Agency) keeps 6,500 high-level radioactive solid waste (in the 200-liter vessel) and about 400 cubic meters of high-level radioactive liquid waste in Tokai which is located 100 km north-east of Tokyo. The former (the solid waste) consists of the end-piece and the cladding of the fuel rods and the latter (the liquid waste) was generated by the failure of the vitrification process. The liquid waste is extremely dangerous and needs to be immobilised as soon as possible. JAEA had planned to vitrify the high-level radioactive liquid waste until 2028, but after the series of accidents they postponed the schedule until 2038. Since the opening of the reprocessing plant in the 1970s, they could not immobilise the nuclear waste for the last 50 years. The main problem of their failure is platinoids in the radioactive waste, which will be explained below. (Kazuyoshi Uraga et al. [16], Energy Frontline [17]) Our solution to this problem is to use Synroc and the professional services by ANSTO (Australian Nuclear Science and Technology Organisation). “Synroc” is the assemblage of multiple ceramics and has been established by Ted Ringwood and ANSTO. (World Nuclear Association [11], ANSTO [10]) ANSTO provides professional services for the immobilisation of radioactive wastes and has experience of similar projects such as Idaho National Laboratory. (Eric Vance et al. [15]) Therefore using Synroc and the professional services by ANSTO we can obtain the satisfactory results immediately. In “Immobilisation of high level radioactive waste” in Research section, we are explaining why Synroc is the best solution.
4. Plutonium in Monju
Currently Japan stores 8.6 tons of plutonium. Among the 8.6 tons, 400 kg of plutonium is stored in Monju, 400 kg is in nuclear power plants as MOX fuel, and the rest of it is kept in the reprocessing plants in Rokkasho and Tokai and in the MOX fuel processing plant in Tokai. (Cabinet Office [18]) Plutonium is extremely harmful for living creatures and needs to be kept safely in the event of earthquakes. Plutonium in Monju must be moved to a safer place before the Nankai Trough Earthquake. If we look upon it as just waste, it is best to immobilise it with Synroc.
5. Middle and long term solution
We proposed the dry cask for the storage of the spent nuclear fuel in the cooling pool and Synroc for immobilisation of the high-level radioactive waste. These are the solutions to survive the Nankai Trough earthquake.
With regards to the long term solution for the nuclear waste, it is impossible to carry out the geological disposal in Japan. Therefore we have no clear endpoint for the handling of the nuclear waste. We have to wait and keep the waste safely until we get better solutions.
With respect to the medium term solution, we still have a lot of things to do. In the first several centuries, FP such as 137Cs and 90Sr are the main source of the radiotoxicity and the radioactivity of HLW. After that period the contribution of MA is 1000 times larger than that of FP. Currently we conduct separation of the spent nuclear fuel to produce the MOX fuel, but we should do it in order to optimise the waste management. It is important to separate the long-lived FP and actinoids from the rest of waste. We should try to transform the long-lived radioactive substances to the stable substances and should continue the study of transmutation. (National Research Council [12]) We should also improve the dry cask storage by the immobilisation of the separated waste and the improvement of the dry cask itself. The skill of separation and transmutation is also useful for handling the melted waste in Fukushima.
We didn’t mention the cost of the proposed solutions. One reason is that we have to solve the problems to survive the Nankai Trough earthquake. Another reason is that there are actually a lot of funds for the nuclear projects in Japan. The reprocessing plant in Rokkasho has used 15 trillion yen (150 billion dollars) for the last 30 years and could not immobilise the HLW. The breeder reactor Monju has used 1 trillion yen (10 billion dollars) and has never run. JAEA submitted a 30 years plan for the dicomission of the unused plant and it was approved. We don’t have time to argue political things, but we should recognise that the nuclear policy in Japan is led by this rotten system.
References
[1] Wikipedia (Dry cask storage), Dry cask storage, https://en.wizkipedia.org/wiki/Japan_Median_Tectonic_Line
[2] United States Nuclear Regulatory Commission, Dry Cask Storage, https://www.nrc.gov/waste/spent-fuel-storage/dry-cask-storage.html
[3] Satoshi Ishikawa et al., Investigation on the current situation of spent nuclear fuel storage in Germany (Japanese), https://confit.atlas.jp/guide/event-img/aesj2019f/2O19/public/pdf?type=in
[4] Chubu Electoric Power, In Pursuit of Greater Safety, https://www.chuden.co.jp/english/energy/hamaoka/provision/
[5] Agency for Natural Resources and Energy, The Current Status of the Nuclear Fuel Cycle to Efficiently Utilize Spent Fuel, https://www.enecho.meti.go.jp/en/category/special/article/detail_186.html
[6] YouTube, How engineers move entire buildings, https://www.youtube.com/watch?v=vKZjUejAjoQ
[7] Wikipedia (Spent fuel pool), Spent Fuel Pool, https://en.wikipedia.org/wiki/Spent_fuel_pool
[8] NHK, Damage estimation of Nankai Trough Earthquake, https://www3.nhk.or.jp/news/html/20250331/k10014762791000.html#anchor-20
[9] The Economic Times, Japan’s “Baba Vanga”, https://economictimes.indiatimes.com/news/new-updates/japans-baba-vanga-predicts-mega-disaster-in-next-three-month/the-rise-of-ryo-tatsuki/slideshow/120157960.cms?from=mdr
[10] ANSTO, Ansto synroc, https://www.ansto.gov.au/products/
[11] World Nuclear Association, Synroc wasteform, https://world-nuclear.org/information-library/appendices/synroc
[12] National Research Council, Nuclear Wastes: Technologies for Separations
and Transmutation, The National Academies Press, Washington, DC, 1996.
[15] Eric R. Vance, Dorji T. Chavara, and Daniel J. Gregg. Synroc develop-
ment—past and present applications. MRS Energy Sustainability, 4:E8,2017
[16] Kazuyoshi Uruga,Takeshi Tsukada,Tsuyoshi Usami, Generation mechanism and prevention method of secondary molybdate phase during vitrification of PUREX wastes in liquid-fed ceramic melter, Volume 57 Issue 4, 2020, https://www.tandfonline.com/doi/full/10.1080/00223131.2019.1691071
[17] Energy Frontline, Vitrification in Reprocessing Plants (Japanese), https://ene-fro.com/article/ef88_a1/
[18] Cabinet Office, Japan’s plutonium management status in 2023 (Japanese), https://www.aec.go.jp/bunya/04/plutonium/20240716.pdf
