Immobilisation of high level radioactive waste
In this section we are going to investigate the immobilisation of high level radioactive waste and consider the solution to the high level liquid waste and solid waste in Tokai.
1. Immobilisation
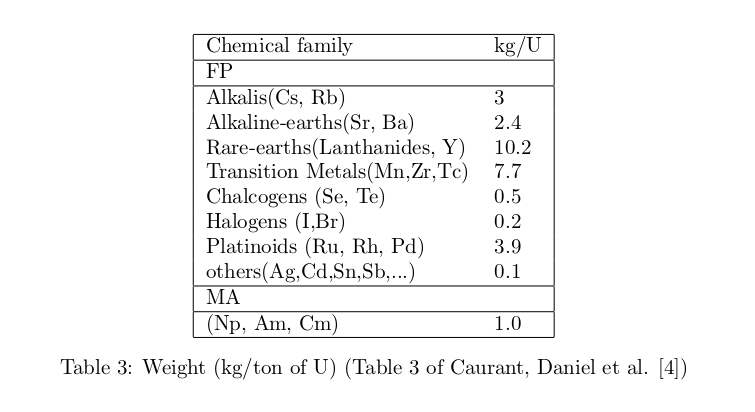
One year operation of a nuclear reactor generates about 100 tons of spent fuel which contains the 95% mass of uranium (93.8% 238U, 0.8% 235U, 0.4% 236U), 1% of plutonium (0.8% 239Pu, 0.2% 240Pu), 4% of fission products (FP) and 0.1% of minor actinides (MA). In reprocessing plants, after retrieving uranium and plutonium the remaining substance is called high-level radioactive waste (HLW) which contains FP, MA and the additional substances (P, Na, Fe, Ni, Cr,..) coming from the retrieving process and the corrosion of the tanks. Table 2 and Table 3 show the half life and the weight of main radionuclides. (Caurant, Daniel et al. [4])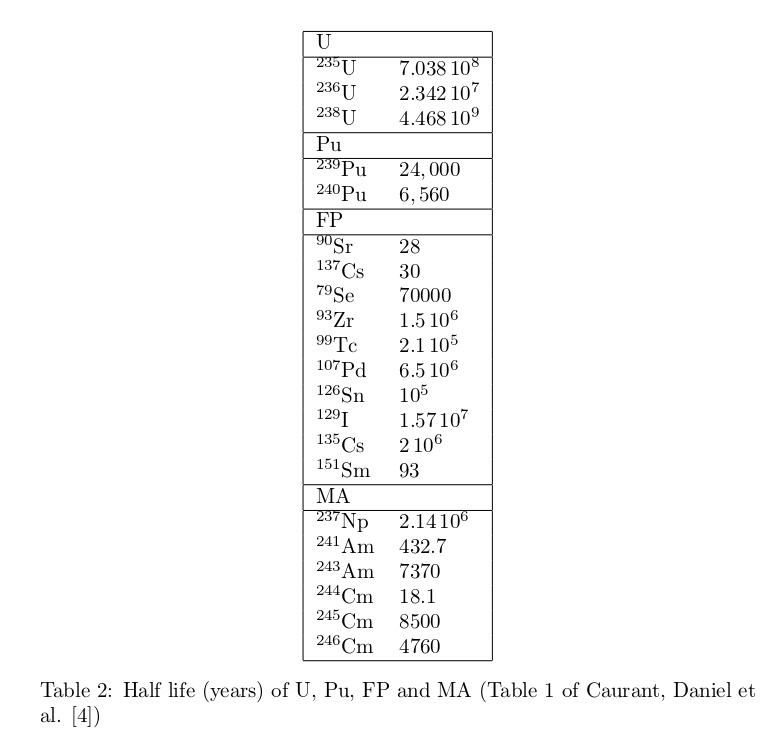
 HLW must be isolated from the biosphere until their radiotoxicity level drops back to that of the natural uranium ore. One way to carry out the isolation is to immobilise HLW in a highly durable matrix. Currently we have three major waste forms: glass, glass-ceramic and ceramic.
HLW must be isolated from the biosphere until their radiotoxicity level drops back to that of the natural uranium ore. One way to carry out the isolation is to immobilise HLW in a highly durable matrix. Currently we have three major waste forms: glass, glass-ceramic and ceramic.
2. Glass
Currently glasses are the main waste forms for the immobilisation of non separated HLW. Borosilicate glasses which mainly consist of SiO2, B2O3 and Al2O3 are preferably chosen because of the good chemical durability and the flexibility to incorporate a variety of chemical elements in HLW. The generation process of the glass waste form is simply melting the wastes with the glass frit in high temperature (1100°C) and cooling them in the canisters. Joule heated ceramic melter (JHCM) which is used in USA, Russia, China and Japan directly heats the melt by Joule effect with the submerged electrodes. The AVM process used in France and UK firstly evaporates and calcines the HLW solution in a rotating kiln (500°C) and then puts the calcined wastes with glass frit into an induction-heated metallic melter (1150°C). (Caurant, Daniel and Majérus, Odile [5])
In the room temperature glass is an amorphous solid which does not posess a long-range order of atomic organization. The structure of the pure silica glass is a continuous random network of SiO4 tetrahedra. Each tetrahedron is linked to four tetrahedra and each oxygen atom is shared between two Si atoms which form strong directional bonds. The Addition of sodium oxide Na2O to the silica glass network decreases the liquidus temperature and the viscosity. The reason is that it transforms two strong Si-O bonds into two weak Na-O ionic bonds. (Figure 4) The further addition of boron oxide B2O3 results in the reinforcement of the glass network. B2O3 participates in the network and generally reduces the weak ionic bonds in the network. Na2O is called a network-modifying oxide and B2O3 is a network-forming oxide. To predict those two different behaviors, the field strength F of a cation M in an oxide is defined as F = Z/d2 where Z is the cation charge of M and d is the mean distance between the cation M and oxygen (in Å (10-10m) unit).
Dietzel Theorem: If F >= 1.5, it is the network-forming oxide and if F <= 0.4, it is the network-modifying oxide. (Caurant, Daniel et al. [4])
If the oxide has high field strength (F >= 1.5) and cannot participate in the network, it tends to create its own network (phase). In the following sentences the glasses mean the borosilicate glasses. 1. alkalis and alkaline-earths
1. alkalis and alkaline-earths
The alkalis and alkalis-earth cations such as Cs+, Rb+, Ba2+, and Sr2+ have low field strength and play the role of network modifier or charge compensator. They are easily dissolved in the glass structure. However, they are also dissolved well in water. When there are poorly soluble anionic entities such as orthophosphate (PO43-) and molybdate (MoO42-), they form the separate phases and deteriorate the overall durability of the glass wasteforms. (Caurant, Daniel and Majérus, Odile [5])
2. Transition metals
2.1. Zirconium
Zirconium Zr (20% of which is 93Zr with half-life of 1.5 106 years) has the high field strength and can participate in the glass network as ZrO6 octahedra. Zirconium can form separate phases such as ZrO2 and ZrSiO4 which are well known for their good chemical durability. (Caurant, Daniel and Majérus, Odile [5])
2.2. Molybdate
Molybdate Mo6+ is a stable fission product and has high field strength. Molybdate is poorly soluble in the glass structure and forms the separate network as MoO42- tetrahedra, called “yellow phase”, which deteriorates the durability of the wasteform. (Caurant, Daniel and Majérus, Odile [5])
3. Platinoids
Platinoids are almost insoluble in the melt and exist as crystalline phase RuO2 and polymetallic (Pd, Rh, Te) alloy. (Caurant, Daniel and Majérus, Odile [5]) They increase the viscosity of the melt and the electrical conductivity. Because of the high density (dPd = 12, dRu02 = 7 compared to dglass = 2.5), they tend to segregate at the bottom of the furnaces. The increased viscosity may block the flow of the melt to the canisters. In JHCM (Joule heated ceramic melter), the increased local electrical conductivity deprives the other part of the Joule heat which is necessary for the vitrification process. It may also cause the risk of short circuit. (Caurant, Daniel et al. [4])
4. Rare earths and actinides
4.1. Lanthanides and actinoides (Am, Cm)
(La, Ce, Pr, Nd) represent more than 90 wt% of lanthanides. Lanthanides (La, Ce, Pr, Nd) and actinoides (Am, Cm) possess similar chemical properties and Lanthanides (La, Ce, Pr, Nd) are used as the MA surrogates in the experiments in the laboratory. Let’s use the notation Ln to represent those elements. Ln3+ has high field strength and is generally well dissolved in the glass network. They form strong Ln-O-Si bonds. (Caurant, Daniel and Majérus, Odile [5])
4.2. Actinoids (U, Pu, Np)
Lighter actinoids (U, Pu, Np) behave differently from the heavier actinoids (Am, Cm). Pu4+ exists in the normal and the oxidizing conditions. Pu3+ occurs only in the reducing conditions. Pu3+ is more soluble in the glass network than Pu4+.
A better method to immobilise plutonium is to use the ceramic wasteform, which will be explained in the ceramic section.
3. Glass-ceramic
Glass-ceramics are composite materials in which crystalline ceramics are distributed homogeneously in the host glass. Separate phases in the glass network would weaken the chemical durability of the wasteform. However as in the case of zirconium shown above some phases have good chemical durability. One strong motivation for the research of glass-ceramics is the idea of the double containment barrier. In this model ceramics contain long-lived radionuclides as the first barrier and they are dispersed in the glass matrix which serves as the second barrier. (Caurant, Daniel et al. [4])
1. Control crystallization
Ceramics in glass can be generated in two steps: nucleation and crystal growth. After mixing the ingredients of glass and ceramics, first we keep the temperature suitable for the nucleation and then we set the temperature for the crystal growth and wait for the development.
In (Caurant, Daniel et al. [4]) an experiment was conducted to form the glass-ceramic for zirconite. At first the glass frit (SiO2, Al2O3, CaO, Na2O) with the zirconite ingredients (TiO2, ZrO2) and the MA surrogate (Nd2O3) are mixed together at 1550°C to form homogeneous glass matrix. Then the mixture was kept at 810°C for 2 hours for the nucleation and at 950 – 1350°C for 2 hours for the crystal growth. After 2 hours of annealing, the glass was converted to glass-ceramic with only zirconite ceramic. In this experiment and in others in the literature, however, the MA surrogate always remains in the residual glass. Therefore the idea of the double containment barrier has not yet been realised with this approach. (Caurant, Daniel and Majérus, Odile [5])
2. Encapsulation
A simpler approach to realise the double containment barrier is to encapsulate the ceramics which already contain the waste into a glass matrix. In (S. Pace et al. [2]) particles of pyrochlore ceramic were encapsulated in a soda borosilicate glass matrix by hot-pressing. Using the relatively low temperature (620°C), the structure of pyrochlore remained unaltered.
4. Ceramic
Ceramics are multiple phase or single phase crystalline materials which exist in geological nature in the earth and other planets such as the moon and mars. Currently ceramics are the de facto standard for the immobilisation of separated long-lived FP and actinoids such as 135Cs, Pu and MA due to the following reasons:
1. Ceramics are generally more durable by several orders of magnitude than borosilicate glasses because of the crystalline structure. The existence of very old ceramics in nature confirms the superior durability.
2. Ceramics are thermally more stable than glasses (Solid glasses have the transition temperature Tg. When the temperature is greater than Tg, the viscosity of the glasses rapidly decreases.)
3. Ceramics can accommodate higher concentration of the waste such as Pu and MA which are generally soluble limitedly in borosilicate glasses.
Ceramics can be produced using several standard methods such as cold pressing and sintering, hot pressing, hot isostatic pressing and spark plasma sintering. (Albina I. Orlova and Michael I. Ojovan [7])
Many types of ceramics have been intensively investigated. We show some examples of the ceramics used for the immobilisation of FP and actinoids.
1. Hollandite
Hollandite Ba(Ti,Al)2Ti6O16 can be used for the immobilisation of Cs. Cs can be accomodated in the tunnels in the framework of (Ti,Al)O6 octahedra. (Figure 5) 2. Zirconolite
2. Zirconolite
Zirconolite CaZrTi2O7 is very durable and has a good capacity to incorporate MA (Np, Am, Cm) and Pu. The structure of zirconolite consists of the Ca/Zr layer (CaO8, ZrO7) and Ti layer (TiO6, TiO5). MA and Pu can be accomodated in the Ca/Zr layer. In the case of Pu we also need to add Gd together to suppress the risk of criticality. (Figure 6)
Synroc (Synthetic Rock) is the assemblage of multiple ceramics. The research started by Professor Ted Ringwood and co workers in 1978 at Australian National University and further developed in collaboration with ANSTO (Wikipedia (Synroc) [1]). Synroc C is a basic formulation and consists of three titanate minerals: hollandite, zirconolite, perovskite, plus minor titanium oxides. It has the capacity to incorporate almost all elements in HLW. (Jostons, A and Kesson, SE [3])
Synroc is mixed with the HLW solution and the mixture is dried and calcined at 750°C to produce a powder. The powder is compressed in the process known as HIP (hot isostatic pressing) at temperature 1150 – 1200°C, which results in a cylinder of dense synthetic rock. (Wikipedia (Synroc) [1])
5. Solution
Our solution for the immobilisation of high-level radioactive waste (solid, liquid) in Tokai is to use Synroc and the professional service by ANSTO.(ANSTO [6])
The reprocessing plants in Tokai and in Rokkasho use JHCM (Joule heated ceramic melter). Due to the problem caused by the platinoids (mentioned above), the operation of the plants stopped.
Glasses are still the main wasteform for the immobilisation of non separated HLW. However, because of the amorphous structure we need to fully understand not only the property of each element but also the relationships among the elements in HLW in order to prevent the undesirable phases from growing in the glass matrix. Ceramics have the crystal structure. They are more thermally stable, more chemically durable, and capable of incorporating more waste than glass matrix. The structure of ceramics does not change before and after the intake of wastes. Therefore ceramics have the better properties and are much easier to handle than glasses.
Sysroc provides a kind of versatile solution. We can adjust the combination of ceramics in Synroc according to the components of the target waste. The high-level radioactive liquid waste in Tokai would contain SiO2 coming from the glass frit and the high-level solid waste would contain metallic substances. We need to adjust the ceramics in Synroc or remove some elements from the waste as the impurities before applying the Synroc process.
References
[1] Wikipedia (Synroc). Synroc. https://en.wikipedia.org/wiki/Synroc.
[2] S. Pace, V. Cannillo, J. Wu, D.N. Boccaccini, S. Seglem, and A.R. Boccac-
cini. Processing glass–pyrochlore composites for nuclear waste encapsula-
tion. Journal of Nuclear Materials, 341(1):12–18, 2005.
[3] A Jostons and SE Kesson, The synroc strategy for hlw management, Min-
eral. Mag. A, 58:458–459, 1993.
[4] Daniel Caurant, Pascal Loiseau, Odile Majérus, V. Aubin-Chevaldonnet,
Isabelle Bardez-Giboire, and Quintas Arnaud. Glasses, Glass-Ceramics
and Ceramics for Immobilization of Highy Radioactive Nuclear Wastes, 04
2009
[5] Daniel Caurant and Odile Majérus, Glasses and Glass-Ceramics for Nu-
clear Waste Immobilization, 01 2021
[6] ANSTO, Ansto synroc, https://www.ansto.gov.au/products/
[7] Albina I. Orlova and Michael I. Ojovan. Ceramic mineral waste-forms for
nuclear waste immobilization. Materials, 12, 2019.
